What is the problem?
When someone has throat cancer, it is sometimes necessary to remove their larynx, the organ that enables air to reach the lungs and food and drink to go separately into the gullet. In such a case, a plastic replacement, or prosthesis, can be inserted but unfortunately this can become clogged by fungi and as a result the prosthesis has to be removed and replaced with a new one. This process may have to be undertaken every 2 to 3 weeks, causing patient concern and taking a great deal of health service time and cost. The following picture shows a comparison of a new and infected prosthesis:
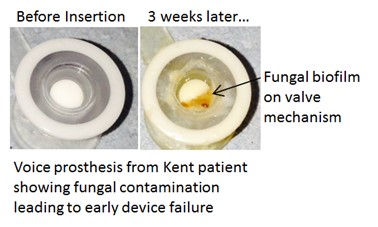
How was the problem tackled?
A multi disciplinary team(MDT) was formed between East Kent Hospitals University NHS Trust and Kent Community Health NHS Foundation Trust and the School of Biosciences at the University of Kent. The remit was to confirm which fungus was causing the problem and then identify ways of overcoming the problem.
What results were achieved?
Candida albicans was confirmed as being the problem fungus. A formal treatment protocol was written to clarify how this condition should be treated. Patients are now asked to apply a small quantity of carefully selected antifungal medication to the voice prosthesis each day. The guidelines also cover what action is most appropriate at every stage of treatment. The MDT reported that in using these guidelines to prevent Candida albicans growth, patient outcome was greatly improved. Those who normally had their voice prosthesis replaced every 2 to 3 weeks as a result of its failure found that this was extended to in-excess of six months. Naturally this makes a huge difference to patients and helps to prevent some of the other complications that can arise when the voice prosthesis fails, e.g. a chest infection. For the clinical staff, patients have to be seen less frequently. Smaller quantities of antifungal medication are used than before the guidelines existed. All this has a favourable effect on the overall cost of treatment and offers significant benefits to patients.
Were other discoveries made?
The MDT has initiated a number of innovative research programmes to find additional improvements. Would it be possible for the patient to monitor the performance of the prosthetic device from their own home using built in wireless technology that can be read using a mobile phone? Would it be possible to re-formulate the silicone material from which the voice prosthesis is made to reduce the likelihood of it being colonised by fungi in the first place? The MDT is making rapid progress in both these areas and it is hoped to announce results by the end of 2018.
What contributions has Kent Cancer Trust made to these developments?
We have spent about £32,000 in support of this project in terms of paying the fees for a PhD candidate for a year and by paying direct laboratory costs for several years. By providing this support, we have enabled the PhD candidate to be trained, thus increasing the number of skilled scientists working in this area.

Would you like more information?
We held a public lecture to announce the results of this work in January 2017 and you can watch that presentation by clicking below.
Is this knowledge kept in Kent?
We are delighted that at the time the results were announced, 28 other NHS Trusts across the United Kingdom had adopted the treatment protocol and that the School of Biosciences would be provided with data and tissue samples from these Trusts so that learnings from across the country can be coordinated. By Decmber 2018, the total number of Hospital Trusts using that treatment protocol had risen to 50! Additionally to that, the treatment protocol has also been adopted in an Australian hospital.
